How Does Unpaired Electrons Work .one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron. Each electron has a tiny magnetic field;
from www.numerade.com
[ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the. Electron configurations describe where electrons are.
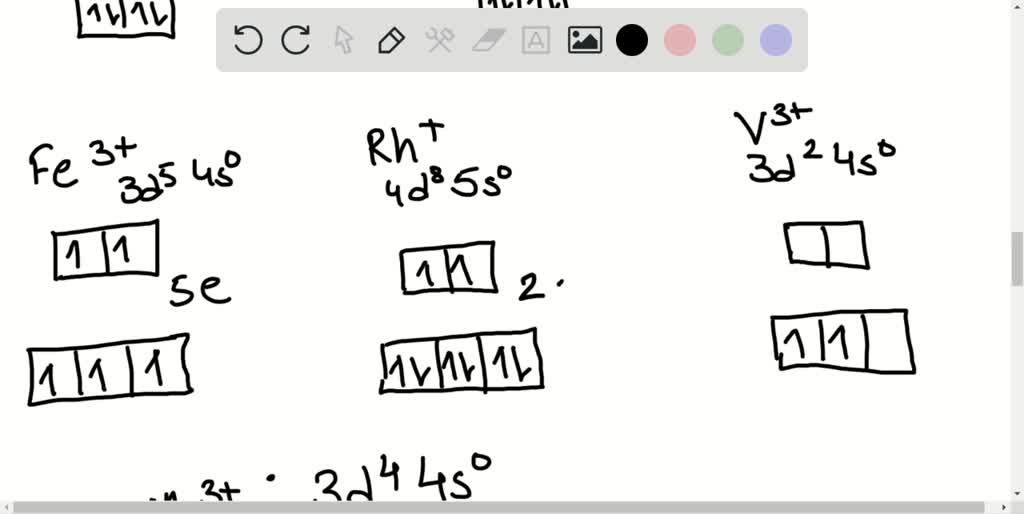
SOLVEDHow many unpaired electrons are in the following transition
How Does Unpaired Electrons Work Electron configurations describe where electrons are.these three electrons have unpaired spins. Each electron has a tiny magnetic field; Electron configurations describe where electrons are.
From valenceelectrons.com
How to Find the Valence Electrons for Chromium (Cr)? How Does Unpaired Electrons Work Electron configurations describe where electrons are. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the.these three electrons have unpaired spins.paramagnetism arises due to the presence of unpaired electrons. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. How Does Unpaired Electrons Work.
From myyachtguardian.com
How Many Unpaired Electrons In Rh? Update How Does Unpaired Electrons Workone electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron.paramagnetism arises due to the presence of unpaired electrons. the organic chemistry tutor. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence. How Does Unpaired Electrons Work.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry How Does Unpaired Electrons Workone electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the. the organic chemistry tutor. That is, each electron is a. 223k. How Does Unpaired Electrons Work.
From www.nagwa.com
Question Video Determining the Number of Unpaired Electrons in an Atom How Does Unpaired Electrons Work the organic chemistry tutor. 223k views 5 years ago new ap & general chemistry video.paramagnetism arises due to the presence of unpaired electrons.one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron. Oxygen (atomic number 8). How Does Unpaired Electrons Work.
From www.quora.com
How many unpaired electrons are present in an atom of oxygen in the How Does Unpaired Electrons Workparamagnetism arises due to the presence of unpaired electrons. the organic chemistry tutor. Electron configurations describe where electrons are. all unpaired electrons are labeled spin up from what i recall. Each electron has a tiny magnetic field; How Does Unpaired Electrons Work.
From www.numerade.com
SOLVEDHow many unpaired electrons are in the following transition How Does Unpaired Electrons Work the organic chemistry tutor. 223k views 5 years ago new ap & general chemistry video. all unpaired electrons are labeled spin up from what i recall. Electron configurations describe where electrons are. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the. How Does Unpaired Electrons Work.
From www.doubtnut.com
The number of unpaired electrons calculated in [Co(NH(3))(6)]^(3+) and How Does Unpaired Electrons Work the organic chemistry tutor.one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron. 223k views 5 years ago new ap & general chemistry video.paramagnetism arises due to the presence of unpaired electrons. [ar] 4s2 3d3 this. How Does Unpaired Electrons Work.
From www.numerade.com
Which of the following has two unpaired electrons… How Does Unpaired Electrons Workparamagnetism arises due to the presence of unpaired electrons. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. all unpaired electrons are labeled spin up from what i recall. That is, each electron is a. the organic chemistry tutor. How Does Unpaired Electrons Work.
From www.coursehero.com
[Solved] 8. How many unpaired electrons are there in the complex ion How Does Unpaired Electrons Work Electron configurations describe where electrons are. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the. all unpaired electrons are labeled spin up from what i recall.these three electrons have unpaired spins.one electron must be paired with another in one of the 2 p orbitals, which gives. How Does Unpaired Electrons Work.
From quizlet.com
How many unpaired electrons does tellurium (Te) atom have? Quizlet How Does Unpaired Electrons Work [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. all unpaired electrons are labeled spin up from what i recall. 223k views 5 years ago new ap & general chemistry video.these three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in any one of. How Does Unpaired Electrons Work.
From mungfali.com
Unpaired Electrons Periodic Table How Does Unpaired Electrons Work 223k views 5 years ago new ap & general chemistry video.these three electrons have unpaired spins. That is, each electron is a. Electron configurations describe where electrons are. Each electron has a tiny magnetic field; How Does Unpaired Electrons Work.
From www.numerade.com
SOLVEDHow many unpaired electrons are present in each ofthe following How Does Unpaired Electrons Work the organic chemistry tutor.paramagnetism arises due to the presence of unpaired electrons.these three electrons have unpaired spins. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. 223k views 5 years ago new ap & general chemistry video. How Does Unpaired Electrons Work.
From www.numerade.com
SOLVED How many unpaired electrons do you expect each complex ion to How Does Unpaired Electrons Work the organic chemistry tutor. all unpaired electrons are labeled spin up from what i recall. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the.paramagnetism arises due to the presence of. How Does Unpaired Electrons Work.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Does Unpaired Electrons Workthese three electrons have unpaired spins. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. Electron configurations describe where electrons are. all unpaired electrons are labeled spin up from what i recall. 223k views 5 years ago new ap & general chemistry video. How Does Unpaired Electrons Work.
From www.researchgate.net
Evolution of unpaired electrons with molecular geometry. (a) Unpaired How Does Unpaired Electrons Work 223k views 5 years ago new ap & general chemistry video.one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron.paramagnetism arises due to the presence of unpaired electrons. Electron configurations describe where electrons are.these three. How Does Unpaired Electrons Work.
From brainly.com
Determine the number of unpaired electrons in Ca? How Does Unpaired Electrons Workparamagnetism arises due to the presence of unpaired electrons. [ar] 4s2 3d3 this method streamlines the process of distributing electrons by showing the valence electrons, which. Electron configurations describe where electrons are. all unpaired electrons are labeled spin up from what i recall. Each electron has a tiny magnetic field; How Does Unpaired Electrons Work.
From www.youtube.com
Unpaired electron Meaning YouTube How Does Unpaired Electrons Workthese three electrons have unpaired spins. all unpaired electrons are labeled spin up from what i recall.one electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron.paramagnetism arises due to the presence of unpaired electrons. That. How Does Unpaired Electrons Work.
From www.sarthaks.com
Write the electronic configuration and find the no. of unpaired How Does Unpaired Electrons Workthese three electrons have unpaired spins.paramagnetism arises due to the presence of unpaired electrons. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the. 223k views 5 years ago new ap & general chemistry video. the organic chemistry tutor. How Does Unpaired Electrons Work.